Evaluation of gonadal macroscopic and microscopic morphometry reveals precocious puberty in Bísaro pig
Paixão G, Esteves A, Carolino N, dos Anjos Pires M, Payan-Carreira R. Evaluation of gonadal macroscopic and microscopic morphometry reveals precocious puberty in Bísaro pig. Reprod Dom Anim 2020;55:1706–1713. https://doi.org/10.1111/rda.13827
Gustavo Paixão 1 | Alexandra Esteves 1 | Nuno Carolino 2,3 | Maria dos Anjos Pires 1 | Rita Payan-Carreira 4
1 Animal and Veterinary Research Centre (CECAV), Universidade de Trás-os-Montes e Alto Douro, Vila Real, Portugal
2 Instituto Nacional de Investigação Agrária e Veterinária, Unidade Estratégica de Investigação e Serviços de Biotecnologia e Recursos Genéticos, Vale de Santarém, Portugal
3 Centre for Interdisciplinary Research in Animal Health (CIISA), Universidade de Lisboa, Lisboa, Portugal
4 MED - Mediterranean Institute for Agriculture, Environment and Development, Universidade de Évora, Évora, Portugal Correspondence Gustavo Paixão, Animal and Veterinary Research Centre (CECAV), Universidade de Trás-os-Montes e Alto Douro, Vila Real 5000-801, Portugal. Email: Este endereço de email está protegido contra piratas. Necessita ativar o JavaScript para o visualizar.
Funding information
European Agricultural Fund for Rural Development, Grant/Award Number: PDR2020-101-031029; European Social Fund, Grant/Award Number: NORTE-08-5369-FSE-000040; Fundação para a Ciência e a Tecnologia, Grant/Award Number: UIDB/CVT/00772/2020
Abstract: Bísaro pig (BP), like many other local breeds reared in extensive non-industrial sys-tems, faces many constraints to comply with the EU welfare regulation, particularly regarding the restrictions to surgical castration. In order to adapt an immunocastra-tion protocol to overtake this issue, a puberty timeline is needed. Using gonadal mor-phometry data from 91 young male BP, this study intended to characterize testicular development, describing the prominent cell types and structures, to ultimately as-sess the age at puberty in male BP through a mixed prediction model. As expected, the relations between several macro and microscopic parameters and their relation with age were as described within the literature. Post-pubertal animals have larger and heavier gonads, lower Sertoli cell density/tubule, higher Leydig cell density and larger seminiferous tubules. Meiosis was firstly seen in 44-day-old animals, elongated spermatids in 70-day-old animals. Complete spermatogenesis was firstly identified in a 90-day-old animal. Spermatozoa were present in the epididymis of 23 animals, aged from 70 to 240 days old, and in the vas deferens of 14 animals (105 to 240 days old). The prediction model inferred that male Bísaro pigs reach puberty between 14 and 17 weeks (3 and 4 months old) and become sexually capable from 15 to 19 weeks (3.5 and 4.4 months old). These parameters confirm the sexual precocity of this breed.
Keywords: boar, epididymis, puberty, spermatogenesis, testis
1 | INTRODUCTION
Bísaro pig (BP) population has grown in numbers in the last de-cade, representing today one of the most important Portuguese livestock breeds (Paixao et al., 2018). Yet, the high-end cured products, which drove this growth, no longer represent the major production of this breed. Difficulties to comply with EU welfare regulations are being pointed out as the main factor involved in this phenomenon. This is especially important when it refers to surgical castration restrictions introduced by the Council Directive 2008/120/EC from December 2008, specifying that surgical cas-tration of pigs shall only be performed with prolonged analgesia and/or anaesthesia. Comprising a large number of smallholders and free-range farms (Paixao et al., 2018), Bísaro producers face many handling constraints to rear young animals up to 14 months of age for cured meat products. Rearing entire animals, with mod-ified feedstuff or improved genetics to lower androstenone and skatole levels in the carcase and thus boar taint, could be pointed as an alternative to physical castration (Backus et al., 2016; Heyrman et al., 2018). Yet, these strategies are less reliable in animals of such age. In local breeds with no consistent breeding programs immunocastration is also suggested as valid alternative to surgical castration (De Briyne et al., 2016; Prunier et al., 2006) even though the effectiveness of this method is yet to prove in non-industrial systems. This aspect can ultimately influence the decision of fattening these animals for cured meat products. Puberty is the process of physical maturation of an animal to be capable of sexual reproduction (Nonneman et al., 2016). In males, it can be defined as the age when physiologically normal sperma-tozoa (SPZ) first appear in the ejaculate (Kumaresan et al., 2011) together with the ability to mate (Karunakaran et al., 2009). The pubertal age depends on genetic and environmental factors, in-cluding intra- and inter-breeds genetic differences, season, social environment and nutritional status (Gethöffer et al., 2018). Besides, the gradual, continuously developing nature of the process makes the exact onset of puberty difficult to establish. Thus, staging and characterizing the spermatogenesis can be performed to facilitate the investigation (Almeida et al., 2006). Notwithstanding, deter-mining the pubertal age is valuable for clinical, breeding and man-agement purposes. For most commercial breeds, the onset of puberty in males occurs between four and seven months of age (Andersson et al., 1999; Ding et al., 2016). Notwithstanding, in these breeds, spermatogenesis is only fully established in six-month-old animals (Malmgren, 1990) where a normal semen composition can also be seen. As per farm-ers’ observations, Bísaro pig is sexually precocious when compared to other commercial breeds locally used (Duroc, Landrace, Large White) and their crosses. Considering the mean values obtained from the herdbook records, gilts have their first farrowing after complet-ing one year old; yet, there are many farrowing records from seven month-old. For boars, breeding records start as early as four months of age (Paixão et al., 2019) with many records at three months old. In spite the farmers’ random observations and breeding records, little is known about the pubertal age of BP. Studying testicular morphometry and assessing spermatogen-esis through the growth phase allow establishing the pubertal pe-riod, and it is a useful tool to delimit puberty in the male. Therefore, this study aims to (a) characterize testicular development eval-uating testicular morphometry through puberty, (b) describe the prominent cell types and structure in testicular sections from de-veloping male Bísaro pigs, (c) determine the age at puberty in male Bísaro pigs based on macroscopical and histological evaluation of both testicular and epididymal tissues. This study will allow to es-tablish and evaluate an early extended immunocastration protocol adjusted to this native breed, reared in extensive, non-industrial systems.
2 | MATERIAL AND METHODS
2.1 | Animals
Testicles and epididymis were collected from 91 male Bísaro pigs ranging in age from one to eight months of age. Piglets were weaned at 28 to 37 days of age, reared in confinement and free-range sys-tems, in heterosexual drifts. Samples were collected post-mortem (n = 26), at the abattoir, and from surgical castration (n = 65), from May 2017 to July 2019, sourced from nine farms. Castrations were conducted as per farm procedures, with a standard anaesthesia and analgesia management protocol, depending on the animal's age. The study was approved by the Animal Care and Ethical Committee of the University of Trás-os-Montes e Alto Douro (Protocol number 657-e-DZ-2018).
May 2017 to July 2019, sourced from nine farms. Castrations were conducted as per farm procedures, with a standard anaesthesia and analgesia management protocol, depending on the animal's age. The study was approved by the Animal Care and Ethical Committee of the University of Trás-os-Montes e Alto Douro (Protocol number 657-e-DZ-2018).
2.2 | Macroscopical parameters
After collection, the testis and epididymis were trimmed, weighed and measured. Both testicles were longitudinally sectioned and tis- sue samples of <1 cm deep were selected from three predetermined areas to cover distinct areas: the cranial (a) and caudal pole (c) of the testicle and a transversal intermediary area (b) comprising the tes-ticular mediastinum (Figure 1). There were also collected transversal sections from the epididymis, at the level of the head (a), corpus (b) and tail (c) and one sample of the deferent duct (d), at the parat-esticular level. The samples were fixed in 10% buffered formalin solution. Testis volume was estimated using the formula: V = 4∕3𝜋 ×l∕2×w∕2×d∕2, where l represents the length, w represents width and d represents depth. Epididymis volume was estimated using the formula: V = l∕4𝜋 ×(w1∕2)2 +l∕2𝜋 ×(w2∕2)2 +l∕4𝜋 ×(w3∕2)2 , whereas w1 corresponds to the width of the head, w2 to body and w3 to the tail.
2.3 | Histological parameters
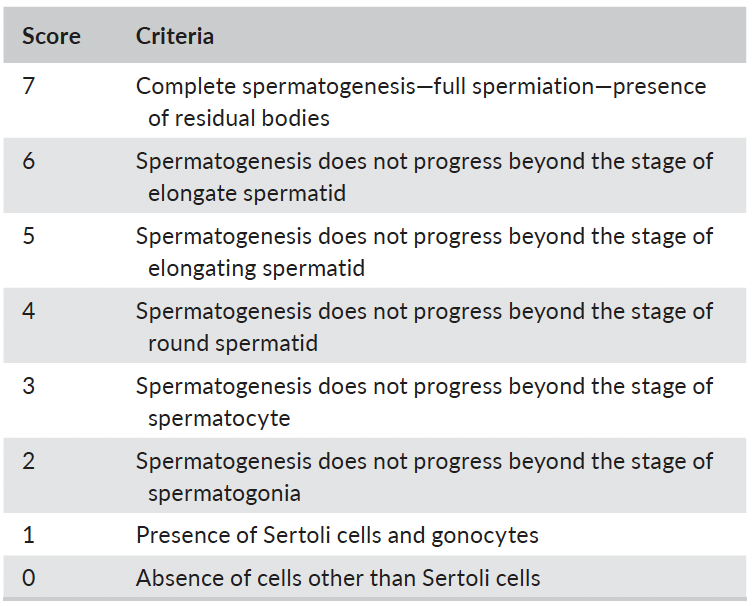
After fixation, tissue samples were routinely dehydrated in an etha-nol series, clarified in xylol and embedded in paraffin wax, followed by microtome sectioning (3–4 µm) and routine haematoxylin–eosin (H-E) staining. Microscopic parameters used for the morphometric studies included the spermatogenesis scoring (SS), the diameter of seminiferous tubule (DiaST), the density of Sertoli (DenS) and Leydig (DenL) cells, the diameter of Leydig cells (DiaLC) and its nucleus (DLN), as well as the ratio between tubular and interstitial areas (RTI). Measurements were made using an image processing software Image J, plugin Fiji (Schindelin et al., 2012) (magnification: ×200 and ×400). To score the spermatogenesis, prominent germinative cell types were peer-assessed by two researchers via light microscopy, using a Nikon E600 microscope (magnification: ×400), in randomly selected cross-sectional profiled seminiferous tubules (n = 100 per testicle) from each area, using a scoring system developed pur-posely for the study, adapted from the Johnsen (Johnsen, 1970) and Yoshida scales (Yoshida et al., 1997) for testicular biopsies. Table 1 summarizes the scoring criteria. The evaluation of the seminiferous tubules was complemented with the microscopic evaluation of the epididymis and the vas deferens (magnification: ×200 and ×400) to identify the presence or absence of SPZ. Histological analysis was unfeasible in samples from four animals as they were not properly fixed.
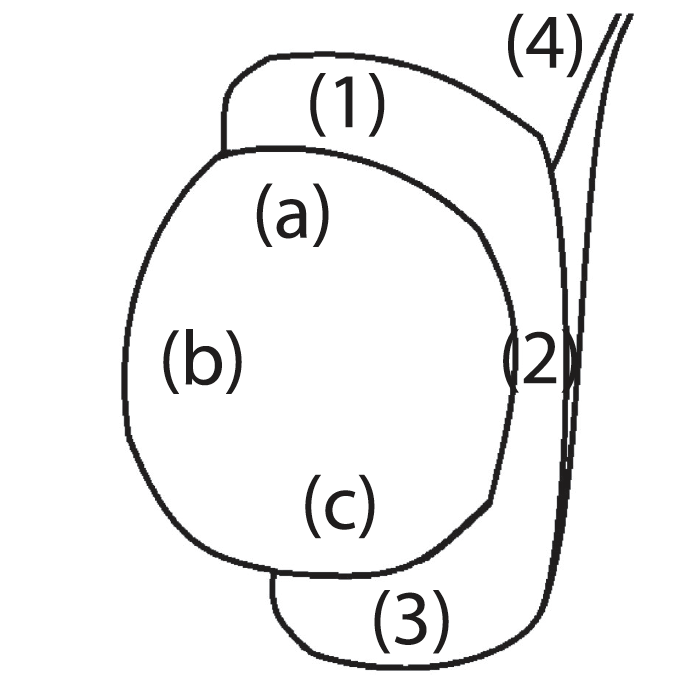
Figure 1 - Tissue sampling areas of the testicle: cranial (a) and caudal pole (c) and a transversal intermediary area (b), the epididymis: head (1), body (2), tail (3) and vas deferens (4)
2.4 | Statistical analysis
Macroscopic and microscopic measurements were grouped by ani-mal. Thus, average values for both organs were calculated through-out all experiment [dataset] (Paixão, 2020). A GLM model was used to predict the animals’ age at puberty considering macroscopic and microscopic effects. This is especially important in variables such as the age, which may be less accurate, due to being recorded by the farmers. The testis and epididymal weight and length, the DiaST, the average SS score and the RTI were included in the model. A logistic fit was then applied to estimate the pubertal age based on the first likely presence of SPZ in the epi-didymis and the vas deferens. Statistical models and analyses were performed using JMP 7 software (SAS Institute Inc., Cary, North Carolina, USA).
3 | RESULTS
Table 2 summarizes the average values for macroscopical param-eters in male Bísaro pigs, at different ages, while Table 3 respects to histological parameters.
Correlations between testicular length, width, depth, weight and volume, were highly positive (r: .891 to 0.995; p < .001; n = 91); simi-lar to correlations between epididymal length, weight and volume (r: .885 to .962; n = 91). In addition, these macroscopic parameters in-creased with age (Figure 2). A positive correlation was also found be- tween DiaLC and DiaLN (r: .636; p < .001; n = 87). Differently, DenS and DenL showed a weak negative correlation (r: −.415; p < .001; n = 87). The DiaST increased on average 1 μm per day the animal got older (R2: .61; r2 = .98; p < .001; n = 87) varying from 52.91 μm to 257.04 μm (Figure 3). RTI showed a similar behaviour, increasing linearly with age (R2: .74; p < .001; n = 87); it varied from 25.36% to 80.52%.
TABLE 1 - Classification for assessing spermatogenesis scoring (SS) in testicular histology
On average, tubules had 16.85(4.41) Sertoli cells per cross section, with a density varying from 2.91 to 99.71 cells/μm2. In fact, the logarithm of DenS decreased when the animals get older (R2: .67 (log); p < .001; n = 87), whereas the logarithm of DenL approximated a second-degree curve (R2: .50; p < .001; n = 87) (Figure 4). Regarding the SS procedure, onset of meiosis, with the presence of spermatocytes (score 3) (Figure 5a), was firstly seen in samples from two 44-day-old animals. Contrastingly, the median and modal scores from both animals, and others with ages comprehended be-tween two and three months old, stayed between 3 and 4. Round spermatids (score 4) to elongated spermatids (score 6) were firstly seen in two animals with 70 days old (score 4) (Figure 5b). Complete spermatogenesis (score 7) was firstly identified in a 90-day-old ani-mal (Figure 5c). None of the tubules scored 0. Spermatozoa were present in the epididymis of 23 animals, aged from 70 to 240 days old (Figure 5d), and in the vas deferens of 14 animals from 105 to 240 days old (n = 91) (Figure 5e). Animals identi-fied with SPZ in the epididymis had a median SS of 6 [5; 6.5], similar to those who also had SPZ in the vas deferens (6 [6; 7]). Differently, the average SS in the group with SPZ in the epididymis was 5.54 (± 1.07) and in the group with SPZ in the vas deferens was 6.17 (± 0.38), respectively. The predicted age when spermatozoa would firstly be found in the epididymis was 103.65 days (ca. 15 weeks) with a 95% CI [94.22, 118.71] (R2: .56; n = 87; p < .001). When this happened, testes were 6.03 cm [5.56, 6.75] length and 3.94 cm [3.55, 4.52] width. For the vas deferens, the model predicted the first pres-ence of SPZ at 120.11 days (ca. 17 weeks) of age with a 95% CI [108.30, 133.70] (R2: .86; n = 87; p < .001). When this happened, on average testes were 7.19 cm [6.50, 8.50] length and 4.76 cm [4.24, 5.69] width.
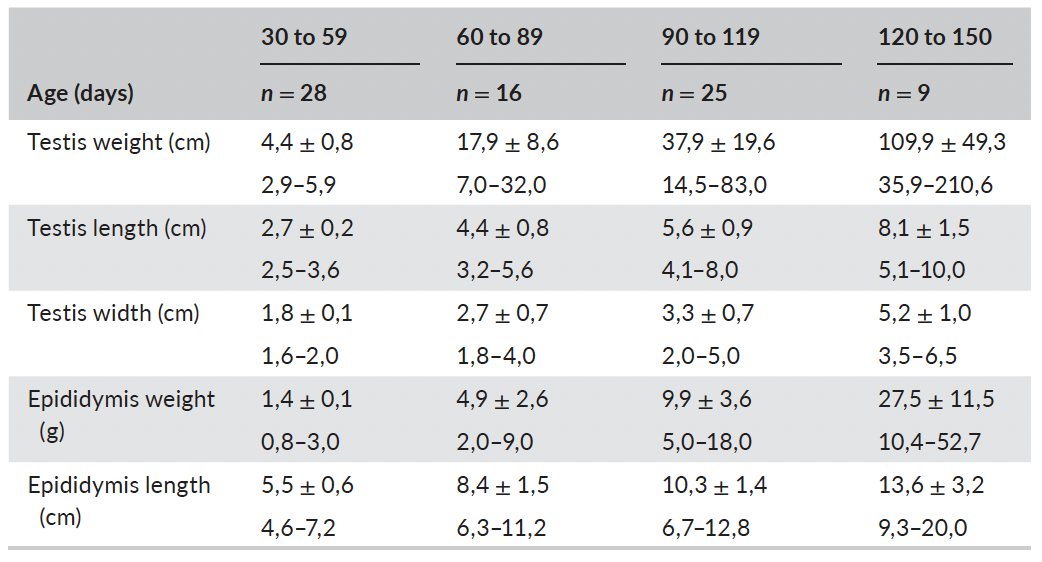
TABLE 2 - Descriptive statistics for macroscopical morphometric parameters (mean ± SD; min - max) at different ages
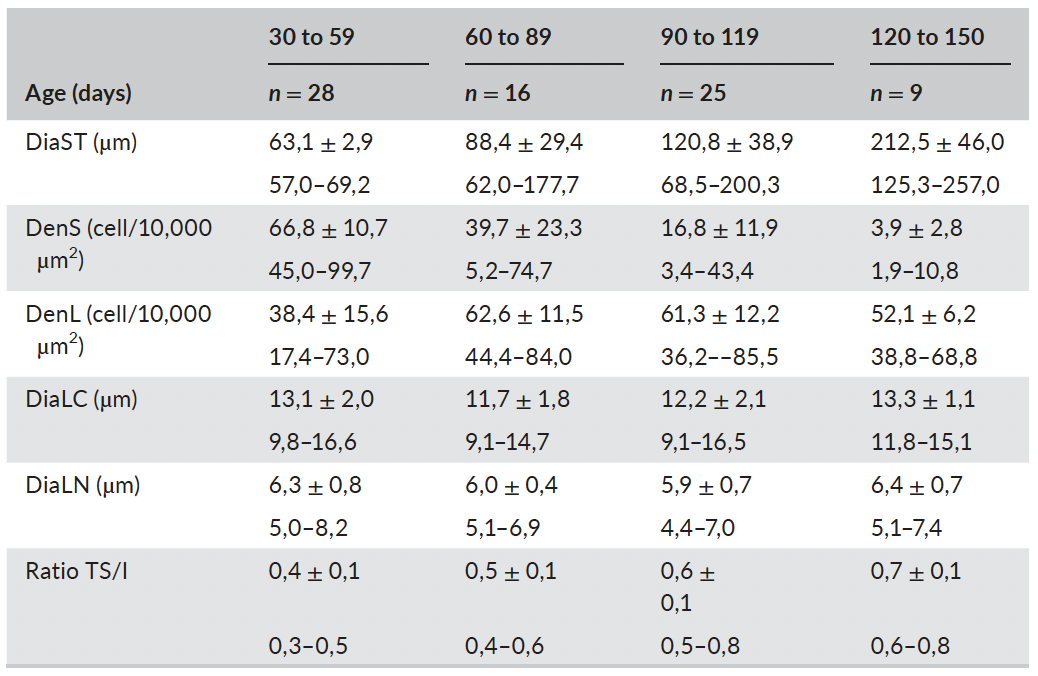
TABLE 3 - Descriptive statistics for histological morphometric parameters (mean ± SD; min - max) at different ages
4 | DISCUSSION
Johnsen and Yoshida's scoring are quantitative methods, normally employed in human medicine, to evaluate testicular capability and analyse testicular histology using a 10 point scale, with a higher score representing a better status of spermatogenesis and a lower score representing more severe dysfunction (Tang et al., 2018). In our study, the score was adapted to accommodate the particularities of pig's testicular morphology and to serve a distinct purpose—staging spermatogenesis through development, as once done by Bohrer for cats (Bohrer, 2016).
As expected, the relations between several macro and microscopic parameters and in relation with age were as described within the liter-ature. In sum, post-pubertal animals have larger and heavier gonads, lower Sertoli cell density, higher Leydig cell density and larger semi-niferous tubules occupying greater areas of the testicular parenchyma (Avelar et al., 2010; Franca et al., 2000; Malmgren et al., 1996).
An overall picture of these correlations shows that great part of the dispersion of values is due to inter-herd variance. Variance between in-dividuals from the same breed, reflect farm management, diet compo-sition and ultimately the animal body growth (Magnabosco et al., 2014). Therefore, additional samples from different farms would have bene-fited the study, and hence the prediction model. Despite the previous, the farm was excluded from the effects list in the age prediction model. Firstly, there were farms with few observations, but mainly, we purposely wanted to exclude any influence that the farm had in the animal's devel-opment, so the predicted age obtained in the model could better reflect the remaining population.
Assessing the pubertal age will always be conjecturing. Nevertheless, sexually mature males must have spermatozoa in the epididymis and ultimately in the vas deferens. These objective param-eters define a clear stage from where we can estimate. From them, other accountable parameters can be estimated, like age. In a time where castration draws much of the pigs’ stakeholders’ attention,
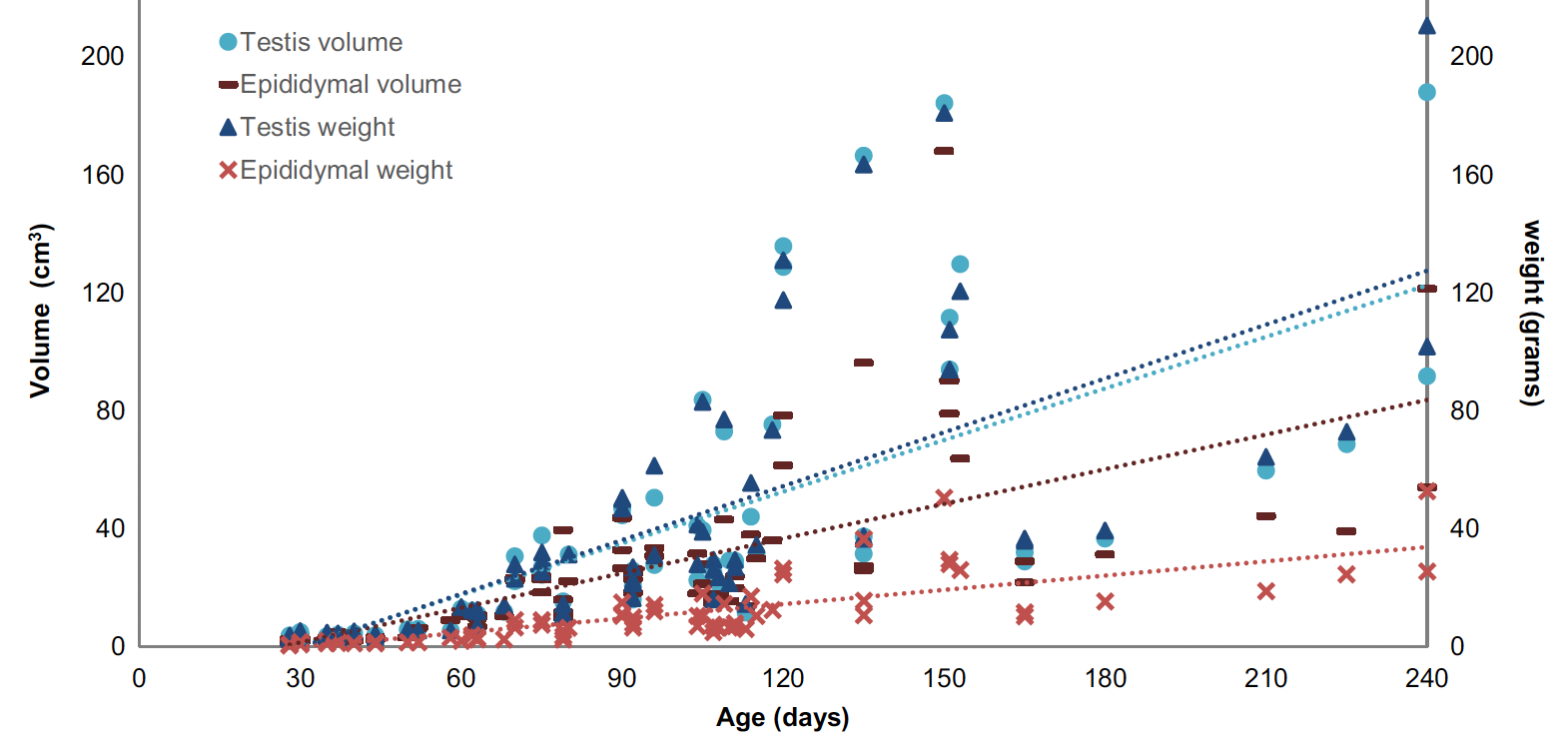
FIGURE 2 - Age-related evolution of testicular and epididymal weight (g) and volume (cm3)

FIGURE 3 - Age-related evolution of seminiferous tubule diameter (µm). Different markers represent individual farms
these estimates may serve the movement in so many practical ways. Animal selection could benefit from late puberty males or early pu-berty gilts. Immunocastration protocols could be optimized, as well as many other management routines may be ameliorated. In sum, de-fining the pubertal age of a given species or breed is essential to their understanding. The prediction model let us infer that male Bísaro pigs reach puberty between 14 and 17 weeks of age (3 and 4 months old) and become sexually capable from 15 to 19 (3.5 and 4.4 months old). Interestingly, BP estimates are very close to those achieved by Kangawa (Kangawa et al., 2016) in microminipigs (18 weeks). These numbers are outdated by some minipigs strains, like Gottingen (8 weeks) (Tab erner et al., 2016) or Naga (Karunakaran et al., 2009), and Asian highly prolific breeds, like Meishan (8 to 12 weeks) (Lunstra et al., 1997; White et al., 1993). It also con-firms some subjective reports from farmers and breeding records from the Breeders association (Paixão et al., 2019). In commer-cial breeds, SPZ are detectable between four and six months and sexual maturity is attained at eight months old in Berkshire pigs and 11 month in Large White (FlorCruz & Lapwood, 1978). Latter puberties are also common in other native breeds, like the Iberian pig (28 weeks in gilts) (Añover, 2009).
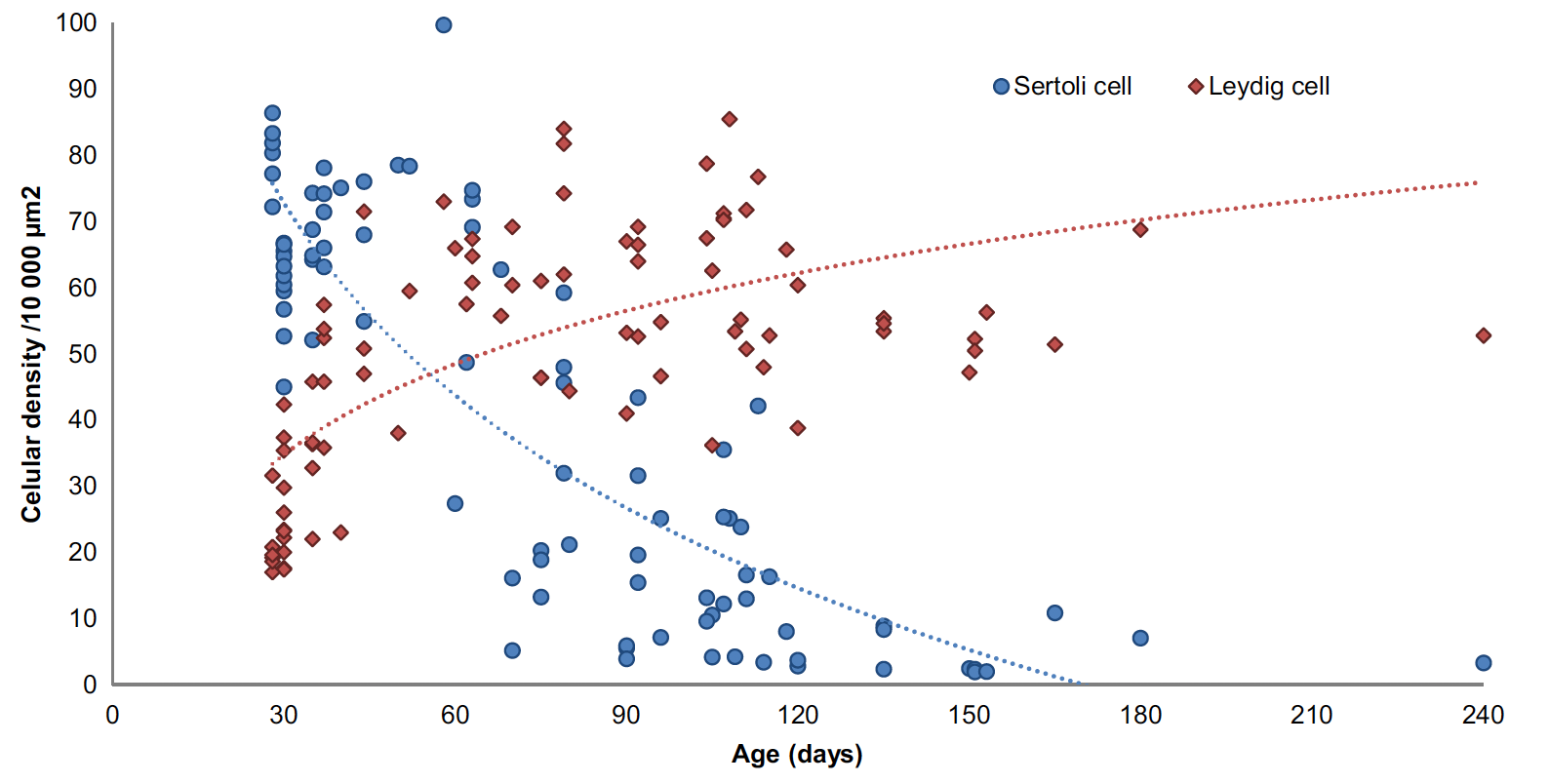
FIGURE 4 - Age-related evolution of Leydig and Sertoli cells density per 10,000 µm2
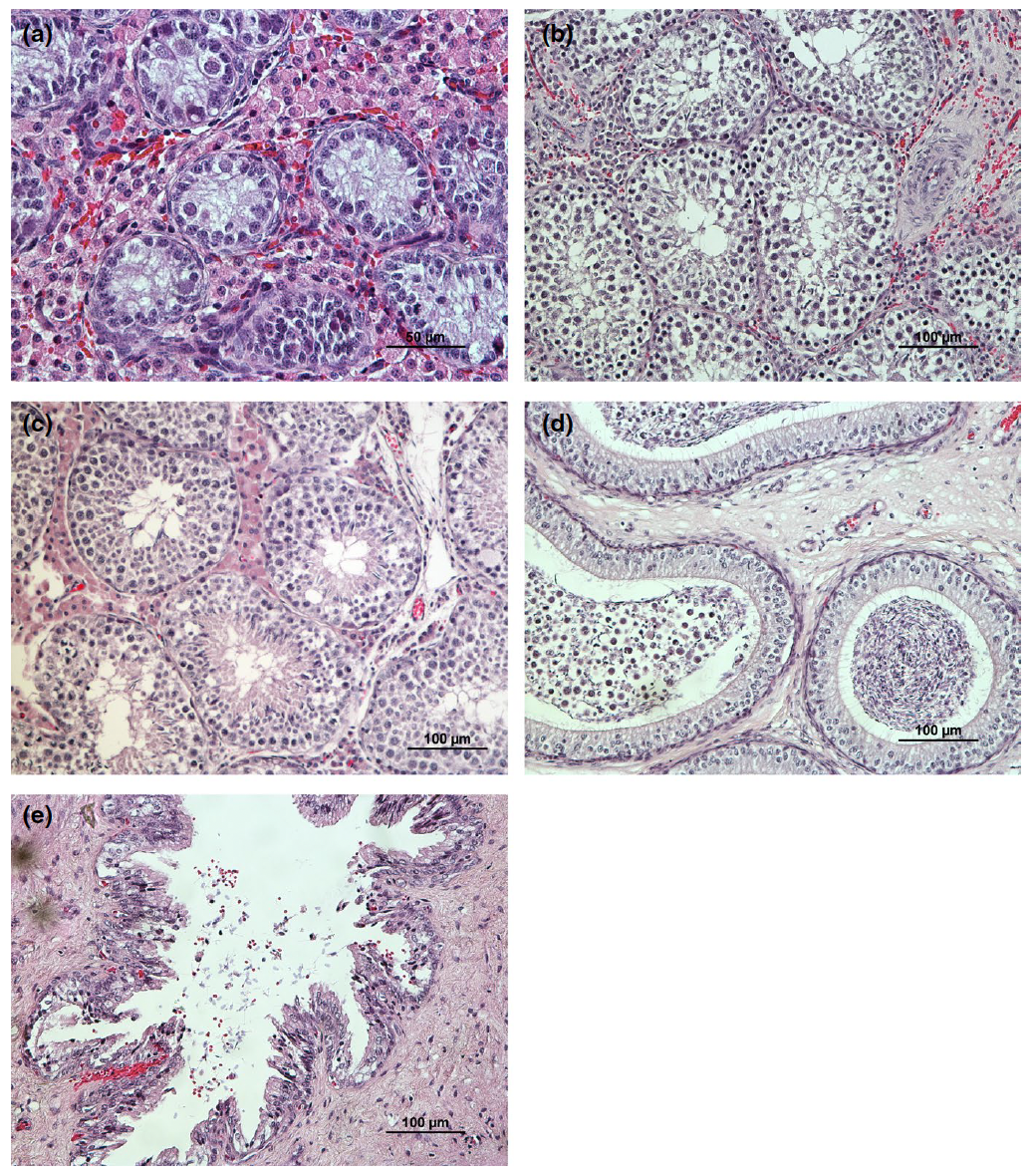
FIGURE 5 - Representative histological features of testis and epididymis from Bísaro pigs at different stages. (a) Testicular cross section from a pre-pubertal 44-day-old Bísaro pig showing germinal epithelium containing spermatogonia, Sertoli cells, primary spermatocytes and a great number of Leydig cells (magnification: ×400). (b) Testicular cross section from a peri-pubertal 70-day-old Bísaro pig showing spermatogonia, spermatocytes and round or elongated spermatids (magnification: ×200). (c) Testicular cross section from a post-pubertal 114-day-old Bísaro pig showing complete spermatogenesis (magnification: ×200). (d) Epididymal head of a 90-day-old Bísaro pig exhibiting cellular debris and spermatozoa inside the lumen (magnification: ×200). (e) Cross section of the vas deferens from a 135-day-old animal (magnification: ×200)
In the current study, the Bísaro had widened the seminiferous tubules to 100 µm at approximately 75 days of age, whereas a four-breed modern composite took 100 days (Ford & Wise, 2011). Moreover, the onset of germ cells proliferation is usually seen at around three-month-old animals (Allrich et al., 1983; Malmgren et al., 1996); in Bísaro pigs, this stage was firstly identified in 1.5-month-old an-imals, and at three-month-old animals, at least more than 50% of their seminiferous tubules were staged accordingly.
5 | CONCLUSIONS
The present study reveals that Bísaro pig is a precocious breed. Macroscopical and histological parameters suggest that male Bísaro pigs reach puberty between three and four months old, when testes are 6 cm length and 4 cm width.
ACKNOWLEDGEMENTS
The authors sincerely thank the Laboratory of Histology and Veterinary Pathological Anatomy from UTAD, particularly its technician Lígia Lourenço. This work was co-funded through the European Agricultural Fund for Rural Development (EAFRD) and the Portuguese Government within the PDR 2020 1.01 Operating Groups initiative, under the project ICas Bísaro (reference no. PDR2020-101-031029). GP holds a PhD grant from the Animal Science Doctoral Program (AniSci), operation number NORTE-08-5369-FSE-000040, co-funded by European Social Fund through the Regional Operational Programme NORTE 2020. This study was also funded by the project UIDB/CVT/00772/2020 supported by the Portuguese Science and Technology Foundation (FCT).
CONFLICT OF INTEREST
None of the authors have any conflict of interest to declare.
AUTHOR CONTRIBUTION
G. Paixão: Conceptualization, Investigation, Methodology, Visualization, Formal analysis, Writing—Original Draft and Writing—Review and Editing. A. Esteves: Project administration, Funding ac-quisition and Writing—Review and Editing. N. Carolino: Validation and Writing—Review and Editing. M. A. Pires: Supervision, Resources, Methodology and Writing—Review and Editing. R. Payan-Carreira: Conceptualization, Methodology, Project adminis-tration, Funding acquisition and Writing—Review and Editing.
DATA AVAILABILIT Y
The data that support the findings of this study are openly available in Mendeley data at http://doi.org/10.17632/ 5t8v7 r8mwm.1
ORCID
Gustavo Paixão - https://orcid.org/0000-0002-9753-8370
Alexandra Esteves - https://orcid.org/0000-0001-6769-1844
Nuno Carolino - https://orcid.org/0000-0001-9079-7380
Maria dos Anjos Pires - https://orcid.org/0000-0001-9473-5283
Rita Payan-Carreira - https://orcid.org/0000-0001-5225-4510
REFERENCES
Allrich, R. D., Christenson, R. K., Ford, J. J., & Zimmerman, D. R. (1983). Pubertal development of the boar: Age-related changes in testicu-lar morphology and in vitro production of testosterone and estradiol-17 beta. Biology of Reproduction, 28(4), 902–909. https://doi.org/10.1095/biolreprod28.4.902
Almeida, F. F. L., Leal, M. C., & França, L. R. (2006). Testis Morphometry, Duration of Spermatogenesis, and Spermatogenic Efficiency in the Wild Boar (Sus scrofa scrofa). Biology of Reproduction, 75(5), 792–799. https://doi.org/10.1095/biolreprod.106.053835
Andersson, H. K., Hullberg, A., Malmgren, L., Lundström, K., Rydhmer, L., & Squires, J. (1999). Sexual maturity in entire male pigs: environ-mental effects, relations to skatole level and female puberty. Acta Agriculturae Scandinavica, Section A - Animal Science, 49(2), 103–112. https://doi.org/10.1080/0906470994 24169
Añover, P. G. (2009). Pubertad, grasa corporal y leptina en cerdas ibéri- cas. Sólo Cerdo Ibérico, , (22), 47–53.
Avelar, G. F., Oliveira, C. F., Soares, J. M., Silva, I. J., Dobrinski, I., Hess, R. A., & Franca, L. R. (2010). Postnatal somatic cell proliferation and seminiferous tubule maturation in pigs: A non-random event. Theriogenology, 74(1), 11–23. https://doi.org/10.1016/j.therio geno logy.2009.12.014
Backus, G., van den Broek, E., van der Fels, B., Heres, L., Immink, V. M., Knol, E. F., Kornelis, M., Mathur, P. K., van der Peet-Schwering, C., van Riel, J. W., Snoek, H. M., de Smet, A., Tacken, G., Valeeva, N. I., & van Wagenberg, C. (2016). Evaluation of producing and marketing entire male pigs. Njas-Wageningen Journal of Life Sciences, 76, 29–41. https://doi.org/10.1016/j.njas.2015.11.002
Bohrer, E. (2016). Determining the Onset of Reproductive Capacity in Free-Roaming, Unowned Cats. (Honors Baccalaureate of Science in Zoology), Oregon State University, Oregon
De Briyne, N., Berg, C., Blaha, T., & Temple, D. (2016). Pig castration: Will the EU manage to ban pig castration by 2018? Porcine Health Management, 2(1), 29. https://doi.org/10.1186/s40813-016-0046-x
Ding, H., Luo, Y., Liu, M., Huang, J., & Xu, D. (2016). Histological and transcriptome analyses of testes from Duroc and Meishan boars. Scientific Reports, 6, 20758. https://doi.org/10.1038/srep20758
FlorCruz, S. V., & Lapwood, K. R. (1978). A longitudinal study of pubertal development in boars investigation of the relationships between go-nadal and epididymal development and plasma luteinizing hormone and testosterone profiles. International Journal of Andrology, 1(1–6), 317–330. https://doi.org/10.1111/j.1365-2605.1978.tb006 03.x
Ford, J. J., & Wise, T. H. (2011). Assessment of pubertal development of boars derived from ultrasonographic determination of testicular diameter. Theriogenology, 75(2), 241–247. https://doi.org/10.1016/j.theriogenology.2010.08.010
Franca, L. R., Silva, V. A. Jr, Chiarini-Garcia, H., Garcia, S. K., & Debeljuk, L. (2000). Cell proliferation and hormonal changes during postnatal development of the testis in the pig. Biology of Reproduction, 63(6), 1629–1636. https://doi.org/10.1095/biolreprod63.6.1629
Gethöffer, F., Pfarrer, C., & Siebert, U. (2018). Histology confirms that macroscopic evaluation of ovaries is a valid method for the assess-ment of the reproductive status in wild boar. Theriogenology, 113, 192–196. https://doi.org/10.1016/j.theriogenology.2018.02.019
Heyrman, E., Millet, S., Tuyttens, F., Ampe, B., Janssens, S., Buys, N., Wauters, J., Vanhaecke, L., & Aluwé, M. (2018). On farm intervention studies on reduction of boar taint prevalence: Feeding strategies, presence of gilts and time in lairage. Research in Veterinary Science, 118, 508–516. https://doi.org/10.1016/j.rvsc.2018.05.008
Johnsen, S. G. (1970). Testicular biopsy score count–a method for regis- tration of spermatogenesis in human testes: Normal values and re-sults in 335 hypogonadal males. Hormones, 1(1), 2–25.
Kangawa, A., Otake, M., Enya, S., Yoshida, T., & Shibata, M. (2016). Histological development of male reproductive organs in micro-minipigs. Toxicologic Pathology, 44(8), 1105–1122. https://doi. org/10.1177/0192623316 673495
Karunakaran, M., Mondal, M., Rajarajan, K., Karmakar, H. D., Bhat, B. P., Das, J., Bora, B., Baruah, K. K., & Rajkhowa, C. (2009). Early puberty in local Naga boar of India: Assessment through epididymal sper-miogram and in vivo pregnancy. Animal Reproduction Science, 111(1), 112–119. https://doi.org/10.1016/j.anireprosci.2008.02.009
Kumaresan, A., Bujarbaruah, K. M., Kadirvel, G., Khargharia, G., Sarma, R. G., Goswami, J., Basumatary, R., Palaniappan, K., & Bardoloi, R. K. (2011). Early sexual maturity in local boars of Northeastern India: Age-related changes in testicular growth, epididymal sperm char-acteristics and peripheral testosterone levels. Theriogenology, 75(4), 687–695. https://doi.org/10.1016/j.theriogenology.2010.10.009
Lunstra, D. D., Ford, J. J., Klindt, J., & Wise, T. H. (1997). Physiology of the Meishan boar. Journal of Reproduction and Fertility. Supplement, 52, 181–193.
Magnabosco, D., Cunha, E. C. P., Bernardi, M. L., Wentz, I., & Bortolozzo, F. P. (2014). Effects of age and growth rate at onset of boar expo-sure on oestrus manifestation and first farrowing performance of Landrace×large white gilts. Livestock Science, 169, 180–184. https://doi.org/10.1016/j.livsci.2014.09.013
Malmgren, L. (1990). Experimentally induced testicular alterations in boars: Hormonal changes in mature and peripubertal boars. Acta Veterinaria Scandinavia, 31(1), 97–107.
Malmgren, L., Rodriguez-Martinez, H., & Einarsson, S. (1996). Attainment of spermatogenesis in Swedish cross-bred boars. Zentralbl Veterinarmed A, 43(3), 169–179. https://doi.org/10.1111/j.1439-0442.1996.tb004 42.x
Nonneman, D. J., Schneider, J. F., Lents, C. A., Wiedmann, R. T., Vallet, J. L., & Rohrer, G. A. (2016). Genome-wide association and identifica-tion of candidate genes for age at puberty in swine. BMC Genetics, 17, 50. https://doi.org/10.1186/s12863-016-0352-y
Paixão, G. (2020). Gonadal morphometry and histology data from Bísaro pigs, In: Paixão, G. (Ed.), Mendeley data. https://doi.org/10.17632/5t8v7r8mwm.1
Paixao, G., Esteves, A., & Payan-Carreira, R. (2018). Characterization of a non-industrial pig production system: The case of Bísaro breed. Revista Brasileira de Zootecnia – Brazilian. Journal of Animal Science, 47. https://doi.org/10.1590/rbz4720170331
Paixao, G., Esteves, A., Payan-Carreira, R., & Carolino, N. (2018). Demographic structure and genetic diversity of the endan-gered Bísaro pig: Evolution and current status. Czech Journal of Animal Science, 63(11), 452–461. https://doi.org/10.17221/113/2018-CJAS
Paixão, G., Martins, Â., Esteves, A., Payan-Carreira, R., & Carolino, N. (2019). Genetic parameters for reproductive, longevity and lifetime production traits in Bísaro pigs. Livestock Science, 225, 129–134. https://doi.org/10.1016/j.livsci.2019.05.010
Prunier, A., Bonneau, M., von Borell, E. H., Cinotti, S., Gunn, M., Fredriksen, B., & Velarde, A. (2006). A review of the welfare con-sequences of surgical castration in piglets and the evaluation of non-surgical methods. Animal Welfare, 15(3), 277–289
Schindelin, J., Arganda-Carreras, I., Frise, E., Kaynig, V., Longair, M., Pietzsch, T., Preibisch, S., Rueden, C., Saalfeld, S., Schmid, B., Tinevez, J.-Y., White, D. J., Hartenstein, V., Eliceiri, K., Tomancak, P., & Cardona, A. (2012). Fiji: An open-source platform for biological-im-age analysis. Nature Methods, 9(7), 676–682.
Taberner, E., Navratil, N., Jasmin, B., Salerno, M., Grambo, B., & Althouse, G. C. (2016). Pubertal age based on testicular and epididymal histol-ogy in Gottingen minipigs. Theriogenology, 86(9), 2091–2095. https://doi.org/10.1016/j.theriogenology.2015.07.030
Tang, W.-H., Zhou, S.-J., Song, S.-D., He, H.-Y., Wu, H., Zhang, Z., Yang, Y.-Z., Zhang, H.-L., Mao, J.-M., Liu, D.-F., Zhao, L.-M., Lin, H.-C., Hong, K., Ma, L.-L., Zhuang, X.-J., & Jiang, H. (2018). A clinical trial on the consistency of bilateral testicular tissue histopathology and Johnsen score: Single side or bilateral side biopsy? Oncotarget, 9(35), 23848–23859. https://doi.org/10.18632/oncotarget.24748
White, B. R., McLaren, D. G., Dziuk, P. J., & Wheeler, M. B. (1993). Age at puberty, ovulation rate, uterine length, prenatal survival and litter size in Chinese Meishan and Yorkshire females. Theriogenology, 40(1), 85–97. https://doi.org/10.1016/0093-691X(93)90343-4
Yoshida, A., Miura, K., & Shirai, M. (1997). Evaluation of seminiferous tubule scores obtained through testicular biopsy examinations of non-obstructive azoospermic men. Fertility and Sterility, 68(3), 514–518. https://doi.org/10.1016/s0015-0282(97)00239-2



